

ISO/IEC 17025 accreditation requires formal documented environment and is viewed by assessors. However, many times the outcome of the tests is not fully documented. There is no doubt that any good analytical laboratory uses qualified analysts, checks the performance of equipment used for testing, and validates analytical methods.

The main difference between good analytical practices and formal accreditation is the amount of documentation to be developed.
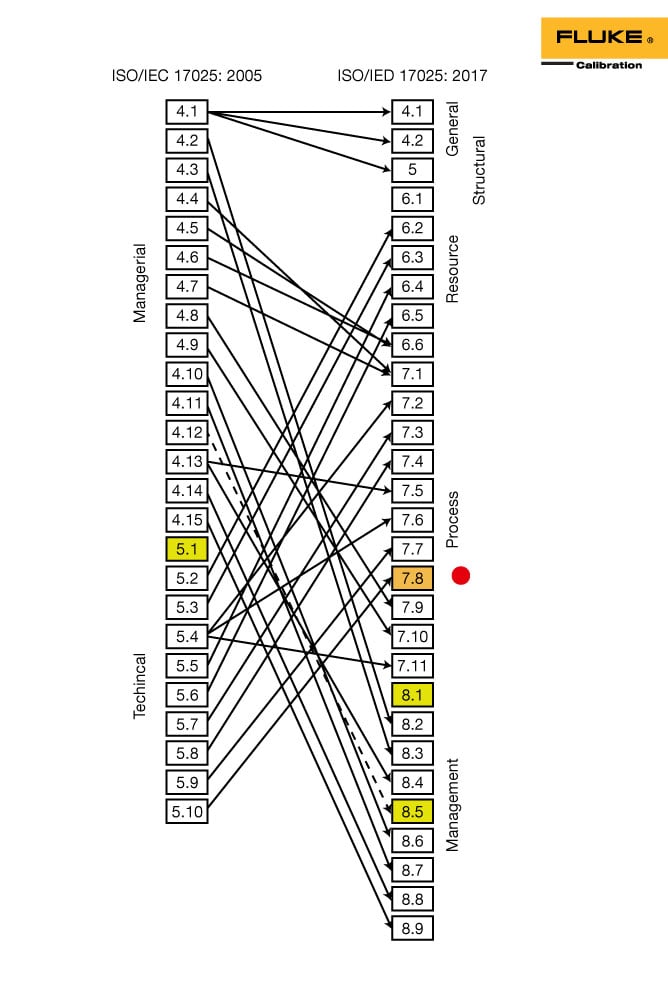
ISO/IEC 17025:2017 is applicable to all laboratories regardless of the number of personnel or the extent of the scope of testing and/or calibration activities. These include, for example, first-, second- and third-party laboratories, and laboratories where testing and/or calibration forms part of inspection and product certification. It is applicable to all organizations performing tests and/or calibrations. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. The subsequent webinars will go into more depth on what is required to meet the requirements each of the elements.ISO/IEC 17025:2017 specifies the general requirements for the competence to carry out tests and/or calibrations, including sampling. It will provide you with an overview of the salient differences and similarities between the old and the new standard. This is the 1st of 3 parts to address the structure and requirements the new standard.
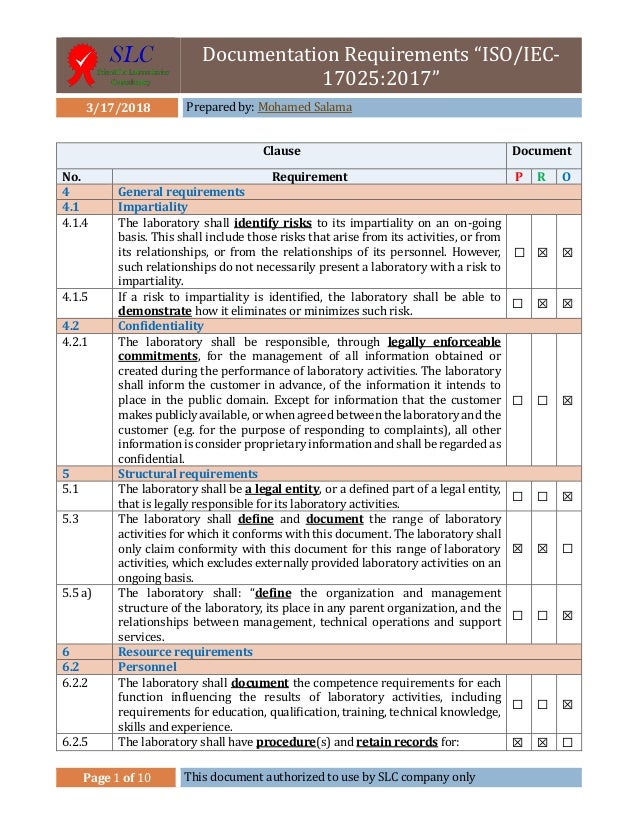
Is everything old new again? What are the new requirements that need to be addressed? This webinar will discuss the changes in the standard and their impact on your QMS. The new version of ISO/IEC 17025 was released on November 19, 2017. Have the rules changed? What are the new requirements of ISO/IEC 17015:2017? How does the new standard impact laboratories that are already accredited and how do you ensure staff adherence and ongoing compliance to minimize corrective actions arising from accreditation audits? Why you should Attend: Some are still struggling to get accredited and even those already accredited still have issues as evidenced by the number of non-conformances cited during the subsequent biannual audits. Many laboratories have successfully developed and implemented a functional quality management system that not only complies with the management and technical requirements of ISO/IEC 17025:2005 but also meets their needs.


 0 kommentar(er)
0 kommentar(er)
